Abstract
Background: Epcoritamab is a novel, subcutaneously (SC) administered, immunoglobulin G1-bispecific antibody that binds to CD3 and CD20, inducing the potent killing of malignant CD20+ B cells. Preclinical findings show that epcoritamab induces T-cell receptor-independent activation and cytotoxic activity of CD4+ and CD8+ T cells against CD20-expressing cells (van der Horst et al, Blood Cancer J 2021). Epcoritamab induces T-cell-mediated cytotoxicity of tumor cells derived from patients with diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL), irrespective of whether tumor cells were exposed to prior anti-CD20 treatment. Epcoritamab employs a different mechanism of action than CD20-targeting monoclonal antibodies, which induce cytotoxicity through Fc-mediated effector functions. Immune-effector cell therapies for relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (NHL), including bispecific antibodies and chimeric antigen receptor T-cell therapy, may require hospitalization after administration, thereby resulting in potential cost and resourcing burden for patients. Recent and ongoing trials have indicated the potential for SC epcoritamab monotherapy administration in the outpatient setting. In the R/R B-cell NHL expansion cohort of the EPCORE NHL-1 study (NCT03625037), cytokine release syndrome (CRS) of any grade was observed in 49.7% of patients; most of these were grades 1 to 2 and mainly occurred following the first full dose of epcoritamab, with median time to onset of 20 hours and median time to resolution of 48 hours (Thieblemont et al, EHA 2022). Importantly, only 2.5% of patients experienced grade 3 CRS events, further supporting the outpatient administration of SC epcoritamab based on its overall safety profile. Because CRS is often observed with T-cell therapy, toxicity-mitigation strategies, including premedication, step-up dosing, and SC administration are a standard aspect of epcoritamab administration. The objective of this study is to evaluate the safety of outpatient administration of the first full dose of epcoritamab in patients with R/R B-NHL with reactive instead of proactive hospitalization.
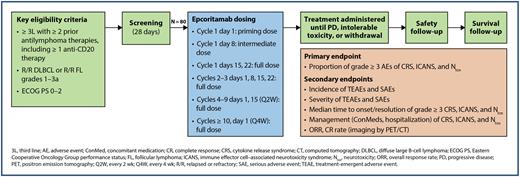
Study Design and Methods: EPCORE NHL-6 is a phase 2, multicenter, open-label study in the United States evaluating the safety of epcoritamab in the outpatient setting without mandatory hospitalization after the first full dose of epcoritamab (NCT05451810). Eligible patients have R/R DLBCL or R/R FL grades 1 to 3a and received at least 2 prior lines of systemic antilymphoma therapies, including at least 1 anti-CD20 monoclonal antibody-containing therapy. Patients must have measurable disease at 1 or more sites and have an Eastern Cooperative Oncology Group performance status of 0 to 2. Approximately 80 patients with R/R DLBCL (n = 40) or R/R FL (n = 40) will be enrolled. Epcoritamab will be administered SC using step-up dosing (priming and intermediate doses on days 1 and 8) followed by full doses on days 15 and 22 in cycle 1 (28 days/cycle) and days 1, 8, 15, and 22 in cycles 2 and 3 (Figure). Additional doses will be administered every 2 weeks for cycles 4 to 9 and every 4 weeks for cycles 10 and beyond until disease progression or at least one of the treatment discontinuation criteria is met. Safety endpoints include the proportion of patients experiencing grade 3 or higher CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), or neurotoxicity (Ntox). CRS and ICANS will be graded using American Society for Transplantation and Cellular Therapy guidelines (Lee et al, Bio Blood Marrow Transplant 2019). Secondary efficacy endpoints include overall response rate and complete response rate (as determined by Lugano 2014 criteria per investigator assessment). Secondary safety endpoints include incidence and severity of treatment-emergent adverse events and serious adverse events as well as median time to onset and resolution of grade 3 or higher CRS, ICANS, and Ntox. Select exploratory efficacy endpoints include duration of response, progression-free survival, and time to response, all of which are determined by Lugano 2014 criteria per investigator assessment, and overall survival. Enrollment will begin in 2022 at approximately 40 US sites.
Disclosures
Sharman:Lilly: Consultancy, Honoraria, Research Funding; Merck: Consultancy; TG Therapeutics: Consultancy, Research Funding; Genentech: Consultancy; Beigene: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Araris Biotech AG: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie Company: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Boccia:Rigel: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Sanofi: Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Regeneron: Speakers Bureau; Genmab: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Doerr:AbbVie: Current Employment, Current equity holder in publicly-traded company. Conte:AbbVie: Current Employment, Current equity holder in publicly-traded company. Bai:AbbVie: Current Employment, Current equity holder in publicly-traded company. Elliott:Genmab: Current Employment, Current equity holder in publicly-traded company. Andorsky:AbbVie: Research Funding; Astra Zeneca: Consultancy, Research Funding; Epizyme: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.